Matter In Our Surroundings
Q1: Define matter.Answer: Anything that occupies space and has mass is known as matter.
Q2: What are the characteristics of matter?
Answer:
- A large number of particles together constitute matter.
- These particles are of very small size.
- Particles of matter have spaces among them.
- Particles of matter are in continuous motion i.e. they possess kinetic energy.
- Particles of matter attract each other i.e. inter particle forces of attraction or intermolecular forces.
Answer: solid, liquid and gas.
Q4: How matter is classified in terms of composition?
Answer: element, compound and mixture.
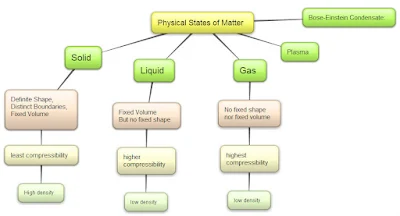
Q5: What are different physical states of matter? Name their properties as well?
Answer:
Q6: Name matter classification in terms of composition.
Answer:
Q7: Define Density.
Answer: The mass per unit volume is called density. Its SI unit is Kgm-3.
Q8. What do you mean by the term Volume?
Answer: The space occupied by a substance is known as volume.
Q9: What do you mean by the following terms:
a. Evaporation
b. Sublimation
c. Condensation
Answer:
a. The process of liquid changes into vapors on heating even below its boiling point is known as evaporation.
b. Sublimation: The changing of the solid directly into vapors on heating and vapors into solid on cooling.
c. The process at which vapors changes into liquid is termed as condensation.
Q11. Define Latent Heat of Fusion and Latent Heat of vaporisation.
Answer: The latent heat of fusion of a substance is the quantity of heat required to convert one unit mass of the substance from solid state to the liquid state at its melting point without any change of temperature.
The quantity of heat required to convert one unit mass of a liquid into vapour at its boiling point without any change of temperature is called its latent heat of vaporisation.
[Another better definition provided by one of our read Mr. Saheel Deshpande is as follows:]
Latent heat of vaporisation: It is the amount of heat consumed when 1 kg of liquid changes into vapor at constant temperature.
Q 12. Define the term Volatile Liquid.
Answer: Those liquids which can change into vapours easily are termed as volatile liquids.
Q 14. Define the following terms:
a. Melting point
b. Freezing point
c. Boiling point
Answer:
a. The temperature at which solid changes into liquid is called its melting point.
b. The temperature at which liquid changes into solid.
c. The temperature at which liquid changes into vapors is termed as its boiling point.
Q 15. (NCERT) Which of the following are matter?
Chair, Air, Love, Smell, Hate, Almonds, Thought, Cold, Cold drinks, Smell of perfume
Answer:Anything which occupies space, mass and can be felt by human senses are called matter. So the things from the above list which are matter are:
- Chair,
- Air,
- Almonds,
- Cold drink
- and Smell of a perfume (It is also considered in matter because it is due to the presence of some volatile substance in air).
(Note: Please see question 55. If you consider smell as sensation then it is not matter. But smell of something e.g. smell of perfume or flower is due to the presence of gaseous matter. For example ammonia has pungent smell and its smell is one of the way to detect presence of ammonia.)
Q 16.(NCERT) Give reasons for the following observations. The smell of hot sizzling food reaches you several meters away, but to get smell from cold food you have to go close.
Answer: Particles present in the matter possess kinetic energy and therefore move constantly. At lower temperature, particles have low kinetic energy and thus move slowly. But as soon as the temperature rises, these particles move faster than when they were in cold. As the particles of hot vapors coming out of hot sizzling food move faster, they can reach several meters away. The particles in the cold food move slowly and thus do not reach us when we are away even a few meters. Therefore we have to go closer to smell cold food.
Q17: Why do gases diffuse rapidly?
Answer: The gases diffuse rapidly because the particles in the gases can freely move in all directions and there is lot of intermolecular space in gases.
Q18:(NCERT) The mass per unit volume of a substance is called density (density=mass/volume). Arrange the following in order of increasing density:
Air, Exhaust from chimneys, Honey, Water, Chalk, Cotton and Iron.
Answer:The order in increasing density is:
- Air
- Exhaust from chimneys
- Cotton
- Water
- Honey
- Chalk
- Iron
Answer: Evaporation is a physical process in which a liquid changes to its gaseous state, at a temperature lower than its boiling point.
Q20: Explain compressibility in gases with an example?
Answer: Liquefied petroleum gas (LPG) cylinders are used in our homes for cooking, contains gases in the compressed state. Similarly, compressed natural gas (CNG) is used as a fuel in vehicles. Large volumes of gases can be compressed in small cylinders and are transported to distant places.
Q21: What is SI unit of temperature? Give mathematical relation also.
Answer: SI unit of temperature is Kelvin. T (K)= T (°C) +273
Q22: Convert the following temperature to Celsius scale:
(a) 300 K
(b) 573 K
Answer: (a) 300K = 300 - 273 = 27 °C
(b) 573 K = 573 - 273 = 300 °C
Q23: Draw diagram to show interconversion among states of matter.
Answer:
Q24: Define transpiration.
Answer: The process of evaporation of water from the aerial parts of plants especially in leaves is called transpiration.
Q25: Which state of matter is most easily compressible?
Answer: Gas state.
Q26: After rains when do rain drops dry away easily– on a cloudy day or on a sunny day? State reason also.
Answer: Rain drops will dry easily on a sunny day. As the temperature is higher in sunny day, evaporation increases. On a cloudy day temperature of the surrounding is low due to humidity while on a sunny day the temperature of the surrounding is high. With an increase of temperature, more number of particles get more kinetic energy to vaporize.
Q27(NCERT): A diver is able to cut through water in a swimming pool. Which property of matter does this observation show?
Answer: The forces of attraction among the particles of water hold it together. But these forces are are not too strong. The force applied by the diver is enough to overcome these forces of attraction. The observation shows the following properties of matter:
- Intermolecular forces in water are not very strong.
- Particles in liquids can be easily displaced from their original position.
- Liquids show reasonably fluidity.
matter in our surroundings class 9Q28: Arrange the following substances in increasing order of intermolecular force of attraction:
matter in our surroundings class 9 notes
matter in our surroundings class 9 ppt
matter in our surroundings wikipedia
matter in our surroundings ppt
matter in our surroundings class 9 questions answers
matter in our surroundings notes
matter in our surroundings class 9 notes pdf
matter in our surroundings class 9 mcq
matter in our surroundings answers
matter in our surroundings activities
matter in our surroundings activities solutions
matter in our surroundings assignment
matter in our surroundings animated
matter in our surroundings questions and answers
matter in our surroundings class 9 answers
matter in our surroundings worksheet with answers
water, sugar, oxygen
Answer: Oxygen < Water < Sugar
Q29: Our own bodies contain examples of all three states of matter. Can you identify these?
Answer:
- Solid: Teeth, bones
- Liquid: blood
- Gas: air (oxygen and carbon di-oxide) that we breathe in-out through lungs.
(a) 25 °C
(b) 0 °C
(c) 100 °C
Answer:
(a) 25 °C : liquid
(b) 0 °C : solid or liquid (if melting process is on way).
(c) 100 °C : gas or liquid (if boiling process is on way).
Q31: What do you mean by change in state?
Answer: Change of substance from one physical state to another is called change of state.
E.g. conversion of ice into liquid water is a change of state from solid(ice) to liquid (water).
Q32: (NCERT) Give reasons to justify:
(a) Water at room temperature is a liquid.
(b) An iron almirah is solid.
Answer:
(a) Water is a liquid at room temperature:
- In water, the intermolecular forces are strong enough to keep its particles bound to each other.
- The melting point of water is below room temperature(so it does not convert into ice).
- Its boiling point is above room temperature (therefore it does not vapourise completely).
- It has fixed shape and definite volume because of strong molecular cohesive forces.
- It is rigid and cannot be deformed easily because of strong molecular forces.
Answer: The speed of particles in any form of matter increases with rise in temperature. Hence the reason.
Q34: How can matter change its state?
Answer: The physical state of any matter can be brought about in the following ways:
- by changing temperature only
- by changing pressure only
- by changing pressure and temperature both.
Answer: Rapid formation and breaking of bubbles in the bulk of a liquid being heated is called boiling.
During boiling particles from the bulk of liquid gain enough energy to get converted to vapour. Therefore it is a bulk phenomenon.
- matter in our surroundings activities
- matter in our surroundings activities solutions
- matter in our surroundings assignment
- matter in our surroundings animated
- matter in our surroundings questions and answers
- matter in our surroundings class 9 answers
- matter in our surroundings worksheet with answers
- matter in our surroundings class 9 assignment
- matter in our surroundings mcq with answers
- matter in our surroundings by wikipedia
- matter in our surroundings value based questions
- matter in our surroundings ncert book
- matter in our surroundings question bank
- matter in our surroundings class 9 board questions
- matter in our surroundings class 9 question bank
- matter in our surroundings fill in the blanks
- matter in our surroundings class 9 value based questions
- questions based on matter in our surroundings
- questions based on matter in our surroundings class 9
- matter in our surroundings class 9 ncert
- matter in our surroundings class 9 ppt free download
- matter in our surroundings class 9 cbse
- matter in our surroundings class 9 extra questions
- matter in our surroundings definition
- matter in our surroundings ppt download
- matter in our surroundings ppt free download
Q36(CBSE): Why does the temperature of a substance remain constant during melting and boiling even when heat is being supplied to it continuously?
Answer: It is because the heat supplied to the substance is used up (or absorbed) in overcoming the intermolecular forces, and therefore, it does not show up as a rise in the temperature.
Following figure shows melting of ice and its vapourisation at 0° C and 100° C respectively.
Q37: Convert the following temperatures:
a. -78.0 °C to Kelvins
b. 775 K to °C
c. 489 K to °C
d. 24 °C to kelvins
Answer: (a) 195 K (b) 502 °C (c) 216 °C (d) 297 K
Q38: Explain with an experiment to show gases do not have fixed shape or volume.
Answer:
Experiment: To show gases do not have fixed shape or volume.
Method:
Conclusion: This shows air has no definite shape or volume. It takes up the shape of the balloon.
Answer: The process in which a liquid changes into its vapour state at temperatures below the boiling point is called evaporation.
Evaporation is an endothermic process i.e. liquid absorbs heat during evaporation. This heat may be provided either by the surroundings or by liquid itself.
When the evaporating liquid takes the required heat from other parts of the liquid, the rest of the liquid cools down. On the other hand, if the liquid takes heat from the surroundings, it causes cooling of the surroundings.
E.g. on a hot day (sunny day), we perspire. When this sweat evaporates, it absorbs the required heat from our body, and we feel cool.
Q40: What causes evaporation?
Answer: During evaporation, the molecules which possess higher kinetic energy leave the liquid and go into the space above the liquid as vapours. The remaining molecules possessing lower kinetic energy are left in the liquid state. Consequently, the average kinetic energy decreases which results in the fall of temperature of the liquid.
Q41: What are the differences between boiling and evaporation?
Answer:
Q42: What factors affect the rate of evaporation?
Answer: Factors that affect the rate of evaporation are:
Answer: The region nearby sea will be more humid. Since evaporation decreases with an increase in humidity. Therefore, evaporation will be faster in area far away from sea.
Q44: Which of the following state does not exist at room temperature conditions:
a) Solids
b) Liquids
c) Gas
d) Plasma
Answer: d) plasma
Q45: Why solids cannot be compressed like gases?
Answer: The particles in solids are so tightly packed that there are no or little inter particle spaces left among them. Therefore solids are not compressible like gases. Gases which have large inter-particle spaces are therefore compressible.
Q46: Why is ice at 273 K more effective in cooling than water at the same temperature?Answer: Ice at 273K or 0 C can absorb a large amount of heat energy as latent heat of fusion to get converted to water state. Therefore Ice at 273K is more effective in cooling.
Q47: List any five physical properties of liquids.
Answer:
Q48: Differences among solids, liquids and gases.
Answer:
States of Matter
Nice chart for FA assignment
Q49: Why is light not considered matter?
Answer: Matter occupies space and has mass. Light has neither of the two and that's why it is not considered as matter. It is taken as form of energy and electromagnetic radiation.
Q50 Why does steam cause more sever burns than boiling water, though both are at same temperature?
Answer: At 100°C, steam carries additional latent heat than boiling water. Therefore steam cause more sever burns.
Q51: What are the ways a gas can be liquified?
Answer: Applying pressure and reducing temperature can liquefy gases.
Q52: What is a dry ice and what are its properties?
Answer: Solid carbon dioxide ( is known as dry ice. It is stored under high pressure. Solid
CO2 gets converted directly to gaseous state on decrease of pressure to 1 atmosphere without coming into liquid state (i.e. sublimes). This is the reason that solid carbon dioxide is also known as dry ice.
It is mainly used as cooling agent because its temperature is very low than water-ice. Dry ice is commonly used in theaters and in movies to produce the effect of fog.
Q53: How vapour is different from gases? Give examples of each.
Answer: In general, those substances which are liquids at room temperature and turn into gas state due to evaporation are called vapours. While the substances which are in gaseous state at room temperature are termed as 'gases'.
e.g. Sulphur, camphor exhibit phase change and turn into vapours. While Carbon Dioxide (CO2), Oxygen (CO2), Nitrogen (N2), Helium (He) exist as gases at room temperatures.
Q54: How heat is transferred when a solid sublimes?
Answer: Certain solids like iodine, naphthalene, solid-CO2 sublimes on heating. Heat is absorbed by the molecules of these solids and is transferred to kinetic energy of molecules. Due to which the molecules show phase change into gaseous state.
Q55: Is smell of garlic or perfume a matter?
Answer: Smell is a sensation. It is an ability of humans or animals how they perceive an odour. However the origin or cause of smell is a chemical (matter). e.g. The smell of garlic is due to the chemical, allicin.
Q56: The mass per unit volume of a substance is called density.(density = mass/volume). Arrange the following in order of increasing density – air, exhaust from chimneys, honey, water, chalk, cotton and iron.
Answer: Exhaust from chimneys, air, cotton, water, honey, chalk, and iron
Q57: Why do solids expand a bit on heating and contract a bit on cooling?
Answer: The solid molecules do not have sufficient inter-molecular (or inter particle) space thus it expands a bit on heating. The inter-particle forces of attraction are very strong which do not let solid particles to leave its mean position. Therefore solid contracts a bit on cooling.
Answer: It is because the heat supplied to the substance is used up (or absorbed) in overcoming the intermolecular forces, and therefore, it does not show up as a rise in the temperature.
Following figure shows melting of ice and its vapourisation at 0° C and 100° C respectively.
Q37: Convert the following temperatures:
a. -78.0 °C to Kelvins
b. 775 K to °C
c. 489 K to °C
d. 24 °C to kelvins
Answer: (a) 195 K (b) 502 °C (c) 216 °C (d) 297 K
Q38: Explain with an experiment to show gases do not have fixed shape or volume.
Answer:
Experiment: To show gases do not have fixed shape or volume.
Method:
- Take two balloons of different shapes. For example One round and one heart shape or cylindrical.
- Fill the balloons with air.
Conclusion: This shows air has no definite shape or volume. It takes up the shape of the balloon.
matter in our surroundings class 9 guide
matter in our surroundings class 9 give reasons
matter in our surroundings hots
matter in our surroundings hindi
matter in our surroundings human need ppt
matter in our surroundings hots questions
matter in our surroundings class 9 in hindi
matter in our surroundings class 9 notes in hindi
matter in our surroundings important notes
matter in our surroundings information
matter in our surroundings introduction
matter in our surroundings is pure
matter in our surroundings class ix
matter in our surroundings class 9 important questions
matter in our surroundings in hindi
matter in our surroundings class 9 intext questions
matter in our surroundings jsunil
matter in our surroundings mcq
matter in our surroundings mcq questions
matter in our surroundings multiple choice questions
matter in our surroundings mind map
matter in our surroundings class 9 mcq with answers
matter in our surroundings class 9 mcq test
matter in our surroundings class 9 mcq questions
matter in our surroundings class 9 meritnation
matter in our surroundings notes class 9
matter in our surroundings ncert solutions
matter in our surroundings ncert
matter in our surroundings notes pdf
matter in our surroundings ncert questions
matter in our surroundings notes ncert
matter in our surroundings of class 9
matter in our surroundings online test
matter in our surroundings online quiz
matter in our surroundings class 9 online test
ppt on matter in our surroundings
project on matter in our surroundings
notes on matter in our surroundings class 9
mcq on matter in our surroundings
ppt on matter in our surroundings free download
matter in our surroundings ppt presentation
matter in our surroundings pdf
matter in our surroundings project
matter in our surroundings powerpoint presentation
matter in our surroundings ppt class 9
matter in our surroundings presentation
matter in our surroundings pictures
matter in our surroundings questions
matter in our surroundings quiz
matter in our surroundings sample questions
matter in our surroundings short questions
questions related to matter in our surroundings
worksheet related to matter in our surroundings
project report on matter in our surroundings
matter in our surroundings solutions
matter in our surroundings summary
matter in our surroundings sample paper
Answer: The process in which a liquid changes into its vapour state at temperatures below the boiling point is called evaporation.
Evaporation is an endothermic process i.e. liquid absorbs heat during evaporation. This heat may be provided either by the surroundings or by liquid itself.
When the evaporating liquid takes the required heat from other parts of the liquid, the rest of the liquid cools down. On the other hand, if the liquid takes heat from the surroundings, it causes cooling of the surroundings.
E.g. on a hot day (sunny day), we perspire. When this sweat evaporates, it absorbs the required heat from our body, and we feel cool.
Q40: What causes evaporation?
OR
Explain evaporation and its cooling effect in terms of kinetic energy of particles.Answer: During evaporation, the molecules which possess higher kinetic energy leave the liquid and go into the space above the liquid as vapours. The remaining molecules possessing lower kinetic energy are left in the liquid state. Consequently, the average kinetic energy decreases which results in the fall of temperature of the liquid.
Q41: What are the differences between boiling and evaporation?
Answer:
| No. | Boiling | Evaporation |
|---|---|---|
| 1 | It occurs at specific temperature. | It occurs at all temperatures. |
| 2 | It requires heat energy to be supplied by external sources. | In general liquid absorbs heat energy from its surroundings. |
| 3 | It takes place throughout the liquid. | It is a surface phenomenon and occurs only at the surface of the liquid. |
| 4 | It is not accompanied by cooling. Temperature of liquid has increased. | In this process the region surrounding the evaporating liquid gets cooler. |
| 5 | It is a rapid and noisy process. | It is slow and quiet process. |
| 6 | Temperature of the liquid remains constant at the boiling point. | Usually the temperature of liquid drops. |
- distinguish between boiling and evaporation
- difference between boiling and evaporation chemistry
- difference between boiling and evaporation in tabular form
- boiling evaporation and condensation
- boiling and evaporation compare and contrast
- boiling water evaporation cold
- compare boiling and evaporation
- contrast boiling and evaporation
- compare boiling and evaporation. (13.4)
- difference between boiling evaporation and condensation
- boiling and evaporation different
- boiling point evaporation difference
- boiling or evaporation definition chemistry
- differentiate boiling and evaporation
- define boiling and evaporation
- distinguish boiling and evaporation
- boiling and evaporation experiment
- boiling water evaporation experiment
- explanation of boiling and evaporation using kinetic theory
Q42: What factors affect the rate of evaporation?
Answer: Factors that affect the rate of evaporation are:
- the surface area exposed to atmosphere i.e. it increases with an increase in surface area.
- temperature. Evaporation increases with increase in temperature.
- humidity. Evaporation decreases with an increase in humidity.
- wind speed. Evaporation increases with an increase in wind speed.
Answer: The region nearby sea will be more humid. Since evaporation decreases with an increase in humidity. Therefore, evaporation will be faster in area far away from sea.
Q44: Which of the following state does not exist at room temperature conditions:
a) Solids
b) Liquids
c) Gas
d) Plasma
Answer: d) plasma
Q45: Why solids cannot be compressed like gases?
Answer: The particles in solids are so tightly packed that there are no or little inter particle spaces left among them. Therefore solids are not compressible like gases. Gases which have large inter-particle spaces are therefore compressible.
Q46: Why is ice at 273 K more effective in cooling than water at the same temperature?Answer: Ice at 273K or 0 C can absorb a large amount of heat energy as latent heat of fusion to get converted to water state. Therefore Ice at 273K is more effective in cooling.
- matter in our surroundings class 9 worksheet
- matter in our surroundings class 9 wikipedia
- www.matter in our surroundings.com
- class 9 chemistry matter in our surroundings worksheet
- matter in our surroundings youtube
- matter in our surroundings class 9 youtube
- chapter 1 matter in our surroundings class 9
- chapter 1 matter in our surroundings
- ch 1 matter in our surroundings
- chapter 1 matter in our surroundings class 9 notes
- chapter 1 matter in our surroundings notes
- chapter 1 matter in our surroundings class 9 ncert
- chapter 1 matter in our surroundings ppt
- chemistry chapter 1 matter in our surroundings
- matter in our surroundings class 7
Q47: List any five physical properties of liquids.
Answer:
- Liquids do not have fixed shape or boundaries.
- They have fixed volume.
- They exhibit fluidity i.e. they can flow.
- Less compressible as compared to gases but higher than solids.
- Lower density as compared to solids.
- Compared to solids, liquids have higher kinetic energy but less than gases.
- The intermolecular forces of attraction are weaker than that of solids.
- Show the property of intermixing i.e. can diffuse.
Q48: Differences among solids, liquids and gases.
Answer:
| Property | Solids | Liquids | Gases |
|---|---|---|---|
| Mass | Definite mass | Definite mass | Definite Mass |
| Shape | Definite shape | No definite shape | No definite shape |
| Volume | Definite Volume | Definite Volume | No Definite Volume |
| Density | Highest | Lower than solids Higher than gases | Lowest |
| Rigidity | Highly Rigid | Less Rigid than Solids | Not rigid |
| Fluidity | Do not flow | From higher level to low level | In all directions |
| Compressibility | Very Low or Cannot be compressed easily | Low | Very High |
| Free Surface | Infinite | One (Upper Surface) | No free surface |
| Diffusion | Cannot diffuse | Few diffuse immediately | Diffuse rapidly |
| Thermal Expansion | Low and Linear | Higher than Solids | Highest |
| Packing of particles | Tightly Packed | Loosely Packed | Very loosely packed |
| Spaces between particles | Very less | Greater space as compared to solids | Greatest |
| Forces of Attraction | Very strong | Less than solids | Least |
| Kinetic Energy(KE) | Very low KE | Higher than solids | Highest KE |
| Movement of Particles | Cannot move freely Vibratory | Can move freely Vibratory, Translatory & Rotatory | Can move freely Vibratory, Translatory & Rotatory |
| Filing a gas container | Do not require container | Requires container | Requires container |
States of Matter
Nice chart for FA assignment
 | |
| States of Matter (credits:James Childs ) |
Q49: Why is light not considered matter?
Answer: Matter occupies space and has mass. Light has neither of the two and that's why it is not considered as matter. It is taken as form of energy and electromagnetic radiation.
Q50 Why does steam cause more sever burns than boiling water, though both are at same temperature?
Answer: At 100°C, steam carries additional latent heat than boiling water. Therefore steam cause more sever burns.
Q51: What are the ways a gas can be liquified?
Answer: Applying pressure and reducing temperature can liquefy gases.
Q52: What is a dry ice and what are its properties?
Answer: Solid carbon dioxide ( is known as dry ice. It is stored under high pressure. Solid
CO2 gets converted directly to gaseous state on decrease of pressure to 1 atmosphere without coming into liquid state (i.e. sublimes). This is the reason that solid carbon dioxide is also known as dry ice.
It is mainly used as cooling agent because its temperature is very low than water-ice. Dry ice is commonly used in theaters and in movies to produce the effect of fog.
Q53: How vapour is different from gases? Give examples of each.
Answer: In general, those substances which are liquids at room temperature and turn into gas state due to evaporation are called vapours. While the substances which are in gaseous state at room temperature are termed as 'gases'.
e.g. Sulphur, camphor exhibit phase change and turn into vapours. While Carbon Dioxide (CO2), Oxygen (CO2), Nitrogen (N2), Helium (He) exist as gases at room temperatures.
Q54: How heat is transferred when a solid sublimes?
Answer: Certain solids like iodine, naphthalene, solid-CO2 sublimes on heating. Heat is absorbed by the molecules of these solids and is transferred to kinetic energy of molecules. Due to which the molecules show phase change into gaseous state.
Q55: Is smell of garlic or perfume a matter?
Answer: Smell is a sensation. It is an ability of humans or animals how they perceive an odour. However the origin or cause of smell is a chemical (matter). e.g. The smell of garlic is due to the chemical, allicin.
Q56: The mass per unit volume of a substance is called density.(density = mass/volume). Arrange the following in order of increasing density – air, exhaust from chimneys, honey, water, chalk, cotton and iron.
Answer: Exhaust from chimneys, air, cotton, water, honey, chalk, and iron
Q57: Why do solids expand a bit on heating and contract a bit on cooling?
Answer: The solid molecules do not have sufficient inter-molecular (or inter particle) space thus it expands a bit on heating. The inter-particle forces of attraction are very strong which do not let solid particles to leave its mean position. Therefore solid contracts a bit on cooling.




Join the conversation